By Mercy Kachenge
Nairobi, Kenya: In response to the public health emergency declared by the Africa Centers for Disease Control and Prevention (Africa CDC) and the World Health Organization (WHO), UNICEF has taken a crucial step to procure Mpox vaccines. This emergency tender aims to control the spread of the Mpox disease.
The UNICEF-led collaboration aims to acquire vaccines for severely affected countries through partnerships with Africa CDC, Gavi, the Vaccine Alliance, WHO, and the Pan American Health Organization, among others.
This collaborative effort will promote vaccine donations from existing stockpiles in affluent nations to curb the ongoing transmission of Mpox, which has resulted in a substantial number of cases and fatalities.
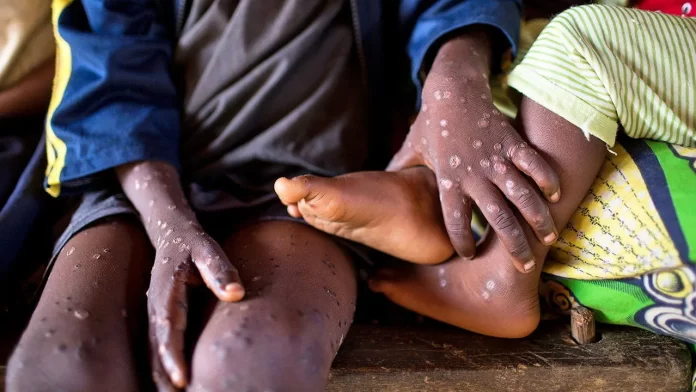
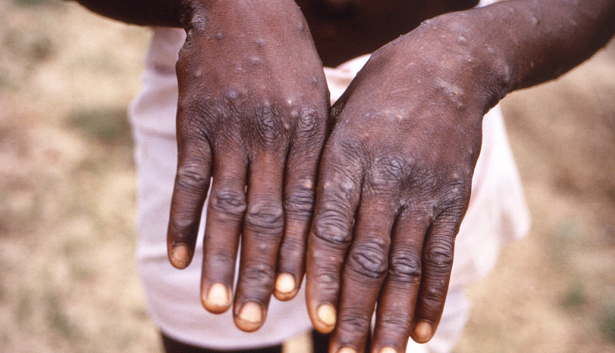
According to the report, the Democratic Republic of the Congo, the epicenter of the crisis, has reported over 18,000 suspected Mpox cases, including 629 fatalities, this year. The report highlights that children account for four out of every five fatalities, emphasizing the gravity of the Mpox situation.
 Leila Pakkala, Director of UNICEF Supply Division, stressed the pressing need to tackle the ongoing shortage of Mpox vaccines and ensure their timely delivery to vulnerable communities.
Leila Pakkala, Director of UNICEF Supply Division, stressed the pressing need to tackle the ongoing shortage of Mpox vaccines and ensure their timely delivery to vulnerable communities.
“There is a pressing need for a universal and transparent allocation mechanism to ensure equitable access to Mpox vaccines,”said Dr.Pakkala.
As stated by Dr. Jean Kaseya,Director General of Africa CDC, echoed the sentiment of timely procurement and distribution of vaccines which is crucial to protecting the most vulnerable populations particularly in the hardest hit regions.
“The emergency tender is a critical step forward in their collective efforts to control and combat the spread of Mpox,”said Dr. Kaseya.
He underscored the Africa CDC commitment in ensuring that vaccines are allocated swiftly and equitably across the continent in partnership with UNICEF,Gavi ,WHO among other key stakeholders.
Dr. Kaseya urged their unified response which is essential in curbing the impact of this public health emergency and safeguarding the health and wellbeing of the communities.
 In a statement, Dr. Maria Van Kerkhove, the World Health Organization’s Incident Manager for the Global Monkeypox Response and acting Director for Epidemic and Pandemic Preparedness and Prevention, emphasized the vital importance of a rapid, coordinated, and equitable response to the current Monkeypox emergency. She noted that such a response is essential for controlling the outbreak and preventing future ones.
In a statement, Dr. Maria Van Kerkhove, the World Health Organization’s Incident Manager for the Global Monkeypox Response and acting Director for Epidemic and Pandemic Preparedness and Prevention, emphasized the vital importance of a rapid, coordinated, and equitable response to the current Monkeypox emergency. She noted that such a response is essential for controlling the outbreak and preventing future ones.
“All of us must act decisively now or risk allowing Mpox to spread further and become an even greater global threat,”said Dr. Maria.
Additionally, Dr. Maria urged that in an interconnected World, the fight against Mpox as with other infectious disease and health threats cannot be waged alone since WHO is glad to partner with Gavi, Africa CDC and other partners and affected countries to get life saving tools to people in need.
Dr. Derrick Sim, the Interim Chief Vaccine Programmes and Market Officer at Gavi, the Vaccine Alliance, stated that various partners are actively collaborating to secure access to vaccine supplies. This collaborative effort aims to facilitate UNICEF’s procurement and delivery of vaccines once Gavi and its partners have secured funding and entered into purchase or donation agreements with manufacturers to meet the most immediate dose requirements.
“Securing access to supply and financing delivering of doses in parallel ensuring countries are ready to administer them are vital actions that need to be conducted rapidly but thoroughly and in a coordinated manner,” said Dr. Derrick.
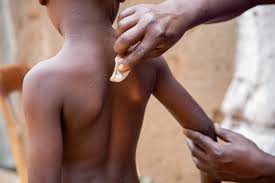
According to WHO,under the emergency tender, UNICEF will set up conditional supply agreements with vaccine manufacturers. This will enable UNICEF to purchase and ship vaccines without delay once countries and partners have secured financing, confirmed demand and readiness, and the regulatory requirements for accepting the vaccines are in place.
WHO is currently reviewing the information submitted by manufacturers on 23 August and is expected to complete its review for Emergency Use Listing by mid-September.
Based on the report, the emergency tenders are designed to secure immediate access to available Mpox vaccines as well as to expand production.Depending on demand, production capacity of manufacturers and funding agreement for up to 12 million doses through 2025 can be established.