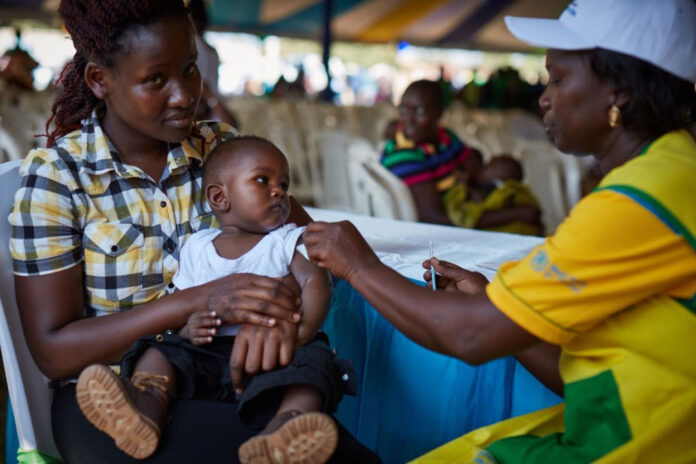
By Henry Owino
Nairobi, Kenya: The fight against malaria has received a boost after the World Health Organization (WHO) approved the second malaria vaccine for use in malaria endemic regions. The vaccine is to be used in children under three years and expected to be a game changer in the management of malaria burden.
The recommendation of a new vaccine, R21/Matrix-M, for the prevention of malaria in children, follows advice from the WHO: Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) and was endorsed by the WHO Director-General following its regular biannual meeting held on 25-29 September, 2023.
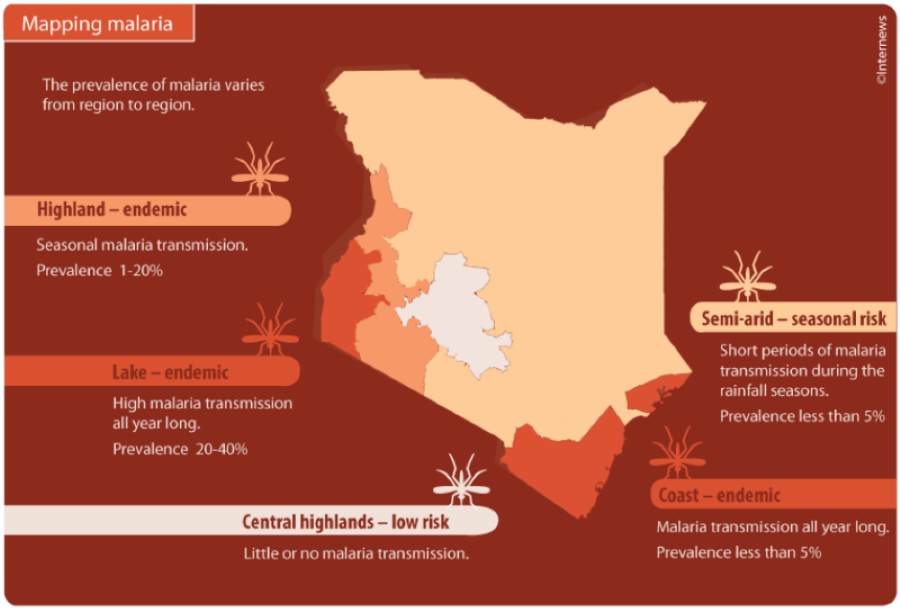
Malaria is a disease that kills at least a child in Africa every minute, putting a lot of burden to parents and government in its management. Currently, Kenya is a place of pride in its research and the development of the vaccine as some trials took place in her coastal region of Kilifi County.
 The green light from WHO for the use of second malaria vaccine adds to the globe anti-malaria arsenal as the world seeks to reduce the impact of the deadly disease.
The green light from WHO for the use of second malaria vaccine adds to the globe anti-malaria arsenal as the world seeks to reduce the impact of the deadly disease.
According to Dr Tedros Ghebreyesus, the Director-General of the WHO, safe and effective vaccines give the world new hope of bringing down one of the oldest diseases known to humanity under control, called malaria.
The vaccine 21R/Matric-M is to be administered to children between aged 5 months to 3 years in what is seen as a game changer to fighting malaria.
The R21 vaccine is the second malaria vaccine recommended by WHO, following the RTS,S/AS01 vaccine, which received a WHO recommendation in 2021. Both vaccines are shown to be safe and effective in preventing malaria in children and, when implemented broadly, are expected to have high public health impact.
Safety and similarity of vaccines
The R21 vaccine works in the same manner as the RTS,S/AS01 vaccine but can be used at much lower dosage, lower cost per dose and manufactured at very large scale.
However, WHO-recommended the two vaccines, R21 and RTS,S, have not been tested in a head-to-head trial. There is no evidence to date showing one vaccine performs better than the other.
 The choice of product to be used in a country should be based on programmatic characteristics, vaccine supply, and vaccine affordability.
The choice of product to be used in a country should be based on programmatic characteristics, vaccine supply, and vaccine affordability.
This second malaria vaccine is as result of researchers’ efforts to improve the current tools use to manage malaria. It is caused by Plasmodium falciparum parasite transmitted by mosquito bites.
In fact, Plasmodium falciparum is the deadliest malaria parasite globally and mostly prevalent in Africa. Kenya played a liberal role in the third phase of trials for the vaccines that were also held in Burkina Faso, Tanzania and Mali.
A total of 4,800 children aged between 5 months and 3 years were enrolled for the clinical trials that approved R21 was 75% effective and safe.
Highly effective before high transmission season
Dr Ghebreyesus said the clinical trials shown the R21 vaccines to be safe and effective. Adding, with the new vaccine, safety monitoring will continue even as the vaccine is rolled out.
“In areas with highly seasonal malaria transmission (where malaria transmission is largely limited to 4 or 5 months per year), the R21 vaccine was shown to reduce symptomatic cases of malaria by 75% during the 12 months following a 3-dose series. A fourth dose given a year after the third maintained efficacy,” Dr Ghebreyesus noted.
“This high efficacy is similar to the efficacy demonstrated when RTS,S is given
seasonally” added Dr Ghebreyesus.
Prof Mainga Hamaluba, Lead Researcher from KEMRI, is the successor of the research and said adoption of the vaccine is a groundbreaking result of teamwork and perseverance.
“The vaccine is a major milestone and a product of long standing commitment and collaboration to control malaria,” Prof Mainga said.
The second vaccine would be administered in 3 doses; one less than recommended dosage of 4 for the first vaccine. Already, the uptake of the first vaccine in Africa has been encouraging with close to 1.7 million doses administered across three countries.

Malaria, a mosquito-borne disease, places a particularly high burden on children in the African Region, where nearly half a million children die from the disease each year globally. In 2022, Africa accounted for 95% of overall disease burden and 96% of death cases recorded.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” said Dr Ghebreyesus, WHO Director-General.
“Demand for the RTS,S vaccine far exceeds supply, so this second vaccine is a vital additional tool to protect more children faster, and to bring us closer to our vision of a malaria-free future.”
Dr Matshidiso Moeti, WHO Regional Director for Africa, emphasized the importance of this
recommendation for the continent, saying “This second vaccine holds real potential to close the huge demand-and-supply gap. Delivered to scale and rolled out widely, the two vaccines can help bolster malaria prevention and control efforts and save hundreds of thousands of young lives in Africa from this deadly disease.”
Demand for malaria vaccines is unprecedented; however, available supply of RTS,S is limited. The addition of R21 to the list of WHO-recommended malaria vaccines is expected to result in sufficient vaccine supply to benefit all children living in areas where malaria is a public health risk.
The RTS,S vaccine will be rolled out in some parts African in early 2024, while the R21 malaria vaccine is expected to become available to the countries in mid-2024.
The world’s largest vaccine manufacturers by dosage, the Serum Institute of India is already lined-up to make more than 100 million doses per year. Plans are underway to escalate it to more than 200 million doses annually.
However, medical practitioners caution that the vaccines should not be seen as alternative to the traditional approach to combating malaria. These include; use of insecticides treated bed-nets, indoor residual spraying, preventive treatment for pregnant women and effective malaria medications.